Understanding Ionization Energy and How to Calculate It
Ionization energy plays a key role in chemistry and physics—it tells us how much energy is needed to remove an electron from an atom. Whether you're studying periodic trends or modeling atomic behavior, understanding ionization energy can help you make sense of how atoms interact.
Our Ionization Energy Calculator makes this complex calculation quick and easy. All you need are three values: the atomic number (Z), the screening constant (S), and the principal quantum number (n). Plug in the numbers, and you'll get a fast and accurate result—no manual calculation required.
What You’ll Need to be able to use our ionization energy calculator
- Atomic Number (Z): The number of protons in the nucleus of the atom—defines the element itself.
- Screening Constant (S): Represents the shielding effect from inner electrons that reduce the effective nuclear charge.
- Principal Quantum Number (n): Indicates the electron’s energy level or shell—important for locating the electron within the atom.
The Formula We Use in ionization energy calculator
This calculator is based on a simplified model from quantum mechanics:
Ionization Energy (eV) = -13.6 × (Z - S)² / n²
This gives the energy in electron volts (eV), a standard unit in atomic physics.
Usage of the calculator : Using calculating techniques for real life applications
Our tool is designed for anyone working with atoms—from students learning the basics to professionals doing advanced research:
- Accurate and Science-Backed: Based on foundational principles in quantum theory.
- Time-Saving: Get your results in seconds—no pen-and-paper math needed.
- 100% Free and Online: Use it from your browser on any device—no signup, no app download.
Real life applications of Ionization Energy
Ionization energy isn't just a textbook topic—it has practical uses in many fields:
- In chemistry labs: Helps explain trends in reactivity across the periodic table (e.g., why alkali metals are highly reactive).
- In astrophysics: Used to identify the composition of stars by analyzing the ionization of gases in stellar atmospheres.
- In plasma physics: Helps model how gases become ionized under extreme conditions, like in fusion experiments.
- In mass spectrometry: Critical for interpreting how molecules break apart and ionize during analysis.
Example of calculating ionization energy. Steps of the calculation are of course included.
Let’s go through a real example step by step:
1. Use the formula:
Ionization Energy = -13.6 × (Z - S)² / n²
2. Assume:
- Atomic Number (Z) = 11 (Sodium)
- Screening Constant (S) = 2.8
- Principal Quantum Number (n) = 3
3. Plug in the values:
Ionization Energy = -13.6 × (11 - 2.8)² / 3²
= -13.6 × 67.24 / 9
= -101.6 eV
4. Final Answer:
The estimated ionization energy for this sodium atom is 101.6 eV.
Keep in mind: the negative sign here represents binding energy—how tightly the electron is held. In practice, ionization energy is often reported as a positive value.
Final Thoughts
Whether you're prepping for a chemistry exam, analyzing stellar data, or just curious about how atoms work, this calculator gives you a fast, clear answer rooted in quantum physics. Try it out next time you're working with atomic models or need a reliable way to estimate ionization energy.
Frequently Asked Questions (FAQs)
What is ionization energy?
Ionization energy is the amount of energy required to remove the most loosely bound electron from a neutral atom in its ground state. It’s usually measured in electron volts (eV) or kilojoules per mole (kJ/mol).
Why is ionization energy important?
It helps explain chemical reactivity and bonding. Elements with low ionization energy (like alkali metals) tend to lose electrons easily and form positive ions, while those with high ionization energy (like noble gases) are more stable and less reactive.
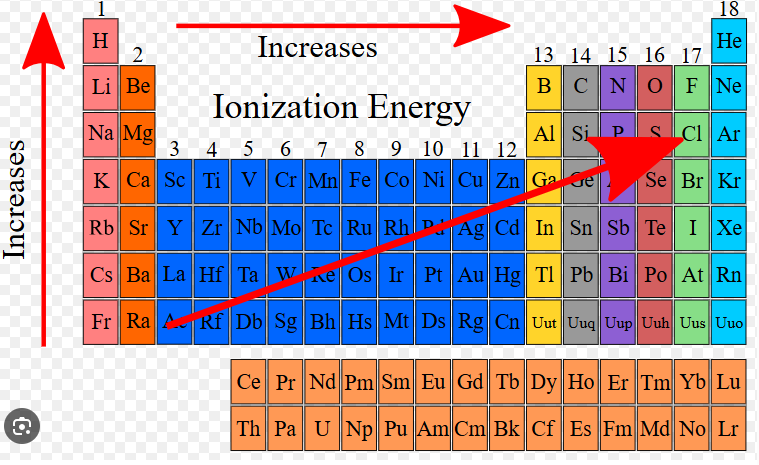
How does ionization energy change across the periodic table?
Ionization energy generally increases across a period (left to right) and decreases down a group (top to bottom). This trend reflects changes in atomic size and nuclear attraction.
What is the screening constant (S) and why does it matter?
The screening constant accounts for the shielding effect from inner electrons. These electrons reduce the effective nuclear charge felt by outer electrons, making it easier to remove them. A higher S value typically lowers the ionization energy.
What does the principal quantum number (n) represent?
The principal quantum number indicates the energy level or shell of the electron being removed. Higher values of n mean the electron is farther from the nucleus and less tightly held, resulting in lower ionization energy.
Is a higher ionization energy always better?
Not necessarily—it depends on the context. For stability, yes. But in chemical reactions, especially when forming compounds or conducting electricity, elements with lower ionization energies often play more active roles.
Can this calculator be used for ions or just neutral atoms?
This calculator is designed for neutral atoms. Calculating ionization energy for already-ionized atoms (like the second or third ionization energy) requires more complex models and updated values for Z, S, and n based on the new electron configuration.
How accurate is this ionization energy calculator?
It provides a solid estimation based on a simplified hydrogen-like model using effective nuclear charge. For precise experimental values, especially for heavier elements, consult spectroscopic data—but for learning and general understanding, it’s very reliable.
Explore related tools: Diffusion Rate Calculator, Flame Temperature calculator, and Phase Change Calculator.